|
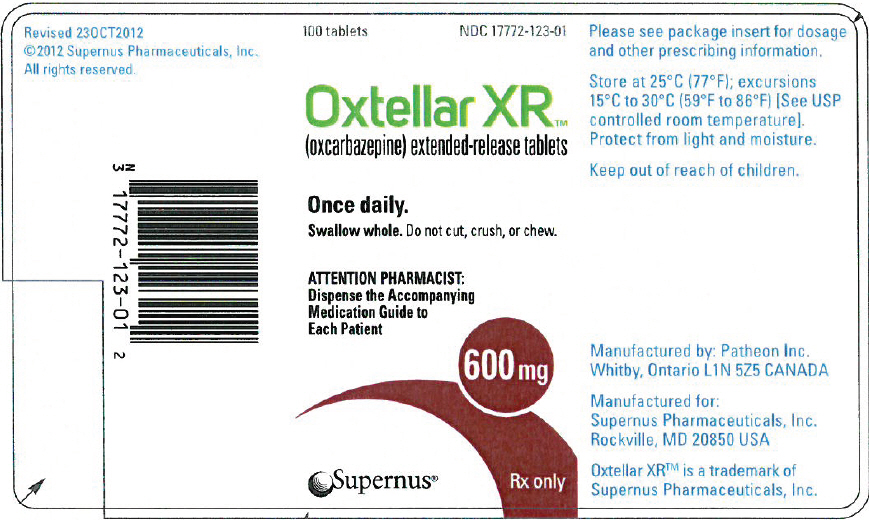
17ma5sa
|
Oral
|
600 mg
|
Extended Release Oral Tablet
|
1
|
24 HR oxcarbazepine 600 mg Extended Release Oral Tablet
|
|
489yfd4
|
Oral
|
150 mg
|
Extended Release Oral Tablet
|
3
|
24 HR oxcarbazepine 150 mg Extended Release Oral Tablet
|
|
73w6jt5
|
Oral
|
300 mg
|
Extended Release Oral Tablet
|
4
|
24 HR oxcarbazepine 300 mg Extended Release Oral Tablet
|
|
c2dqtc3
|
Oral
|
15000 mg
|
Oral Suspension
|
13
|
Oxcarbazepine 60 mg/mL Oral Suspension
|
|
ffhsvah
|
Oral
|
150 mg
|
Extended Release Oral Tablet
|
1
|
24 HR oxcarbazepine 150 mg Extended Release Oral Tablet
|
|
qff3xha
|
Oral
|
300 mg
|
Oral Tablet
|
53
|
Oxcarbazepine 300 mg Oral Tablet
|
|
vwcynsz
|
Oral
|
600 mg
|
Oral Tablet
|
69
|
Oxaprozin 600 mg Oral Tablet
|
|
xspy3rx
|
Oral
|
300 mg
|
Oral Suspension
|
5
|
Oxcarbazepine 60 mg/mL Oral Suspension
|
|
z6p0ckq
|
Oral
|
150 mg
|
Oral Tablet
|
47
|
Oxcarbazepine 150 mg Oral Tablet
|