|
36h2nxk
|
Oral
|
2 mg
|
Sublingual Tablet
|
25
|
Buprenorphine 2 mg Sublingual Tablet
|
|
3tcr9de
|
Oral
|
0.6 mg
|
Buccal Film
|
1
|
Buprenorphine 0.6 mg Buccal Film
|
|
51ezpcw
|
Oral
|
0.07 mg
|
Buccal Film
|
1
|
Buprenorphine 0.075 mg Buccal Film
|
|
6n1yyet
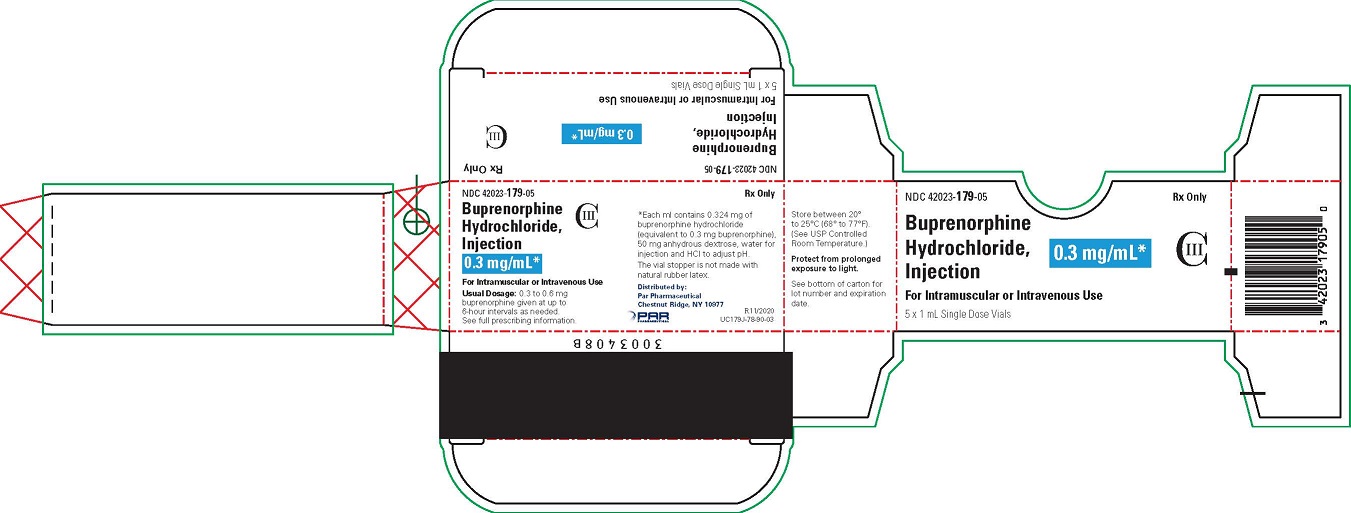
|
Injectable
|
0.3 mg
|
Cartridge
|
1
|
Buprenorphine 0.3 mg/mL 1 mL Cartridge
|
|
7ehs0pq
|
Oral
|
8 mg
|
Sublingual Tablet
|
39
|
Buprenorphine 8 mg Sublingual Tablet
|
|
c4xzdra
|
Oral
|
0.45 mg
|
Buccal Film
|
1
|
Buprenorphine 0.45 mg Buccal Film
|
|
c70j0m6
|
Oral
|
0.75 mg
|
Buccal Film
|
1
|
Buprenorphine 0.75 mg Buccal Film
|
|
jja2v06
|
Oral
|
0.15 mg
|
Buccal Film
|
1
|
Buprenorphine 0.15 mg Buccal Film
|
|
n1p0ta9
|
Oral
|
0.3 mg
|
Buccal Film
|
1
|
Buprenorphine 0.3 mg Buccal Film
|
|
vt0g2k0
|
Tablet
|
2 mg
|
Tablet
|
1
|
Buprenorphine HCl 2 mg Tablet
|
|
yrat4k9
|
Oral
|
1 mg
|
Buccal Film
|
1
|
Buprenorphine 0.9 mg Buccal Film
|