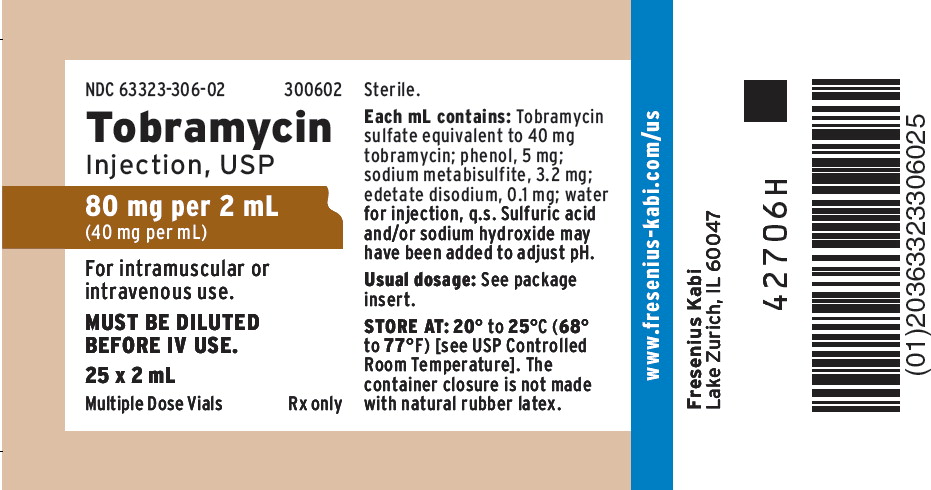
NDC 63323-0306-02 25
Tobramycin 40 mg/mL Vial [Nebcin]
| Supplier | Fresenius Kabi USA, LLC |
|---|---|
| Availability Status | No Availability Issues Detected |
| Status Description |
This product is either shipping normally or there is insufficient availability data to declare a disruption. |
| QUMI Product Code | bv8xv17 (QuicksortRx Universal Medication Identifier) |
| NDC | Package Count | Supplier | Description | Status | Updated |
|---|---|---|---|---|---|
| 25 | Hospira, Inc. | Tobramycin 40 mg/mL Vial [Nebcin] | No Availability Issues Detected | ||
| 25 | Baxter Healthcare Corporation | Tobramycin 40 mg/mL Vial [Nebcin] | No Availability Issues Detected | ||
| 25 | Mylan Institutional LLC | Tobramycin 40 mg/mL Vial [Nebcin] | No Availability Issues Detected | ||
| 25 | Gland Pharma Limited | Tobramycin 40 mg/mL Vial [Nebcin] | No Availability Issues Detected |
| RXCUI: | 597823 |
|---|---|
| Dosage Form: | Vial |
| Dosage Route: | INJECTABLE |
| Strength: | 80 |
| Measure: | mg |
| ANDA: | ANDA065122 |
| Generic Description: | TOBRAMYCIN SULFATE |
| DEA: |