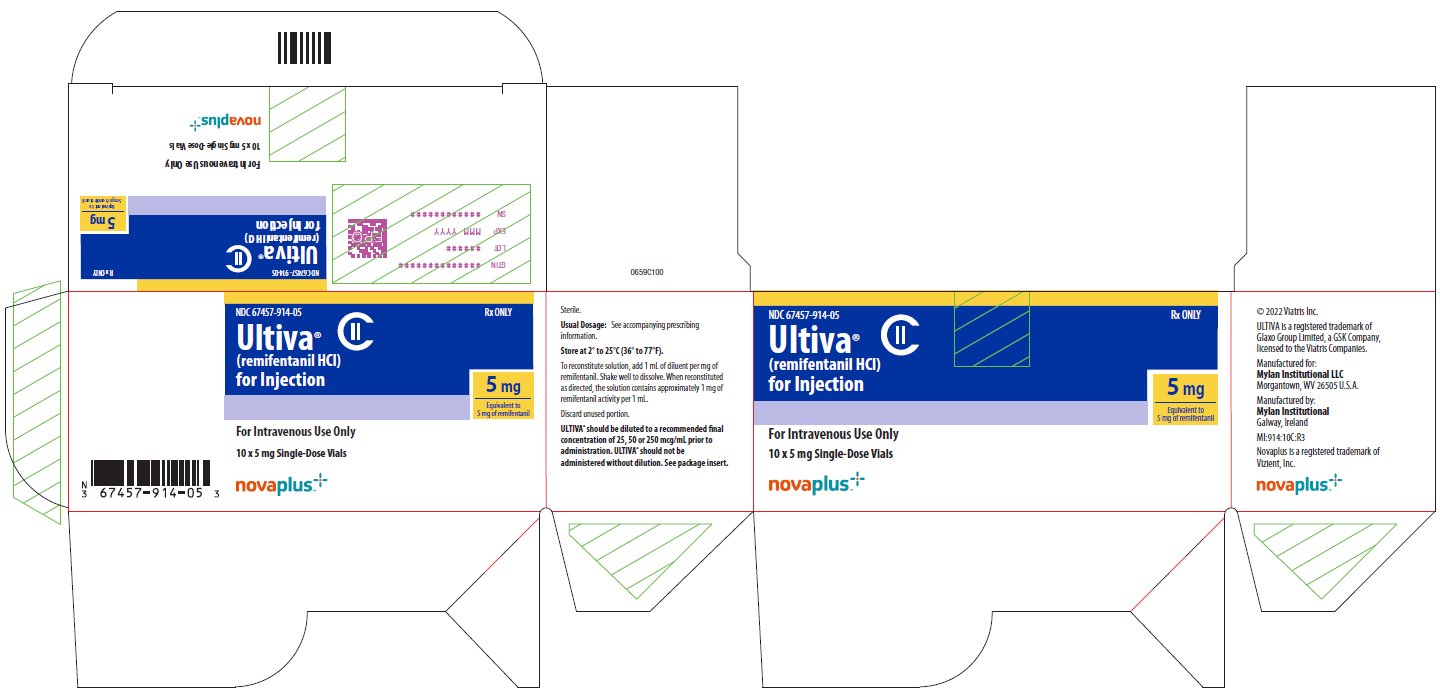
NDC 67457-0914-05 10
Remifentanil 5 mg Vial [Ultiva]
| Supplier | Mylan Institutional LLC |
|---|---|
| Availability Status | No Availability Issues Detected |
| Status Description |
This product is either shipping normally or there is insufficient availability data to declare a disruption. |
| QUMI Product Code | h5w80rp (QuicksortRx Universal Medication Identifier) |
| NDC | Package Count | Supplier | Description | Status | Updated |
|---|---|---|---|---|---|
| 10 | Hikma Pharmaceuticals USA Inc. | Remifentanil 5 mg Vial [Ultiva] | ASHP Shortage + QS Availability Issues | 2024-07-25 | |
| 10 | Fresenius Kabi USA, LLC | Remifentanil 5 mg Vial [Ultiva] | ASHP Shortage + QS Availability Issues | 2024-08-10 | |
| 10 | Mylan Institutional LLC | Remifentanil 5 mg Vial [Ultiva] | QuicksortRx NDC Availability Disruption | 2024-07-31 |
| RXCUI: | 1729710 |
|---|---|
| Dosage Form: | Vial |
| Dosage Route: | INJECTABLE |
| Strength: | 10 |
| Measure: | mg |
| ANDA: | NDA020630 |
| Generic Description: | REMIFENTANIL HCl |
| DEA: | 2 |