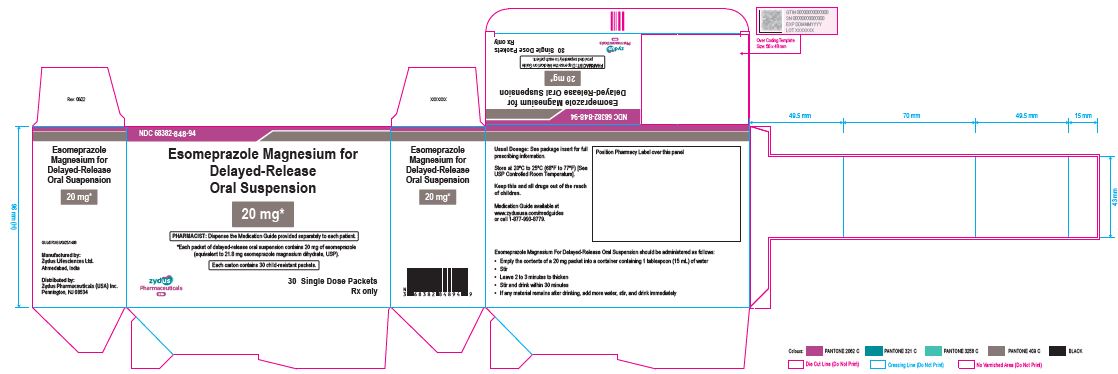
NDC 68382-0848-94 30
Esomeprazole 20 mg Granules for Oral Suspension [Nexium]
| Supplier | Zydus Pharmaceuticals USA Inc. |
|---|---|
| Availability Status | No Availability Issues Detected |
| Status Description |
This product is either shipping normally or there is insufficient availability data to declare a disruption. |
| QUMI Product Code | eaqhkjr (QuicksortRx Universal Medication Identifier) |
| NDC | Package Count | Supplier | Description | Status | Updated |
|---|---|---|---|---|---|
| 30 | AstraZeneca Pharmaceuticals LP | Esomeprazole 20 mg Granules for Oral Suspension [Nexium] | No Availability Issues Detected | ||
| 30 | Cipla USA Inc. | Esomeprazole 20 mg Granules for Oral Suspension [Nexium] | No Availability Issues Detected | ||
| 30 | Zydus Lifesciences Limited | Esomeprazole 20 mg Granules for Oral Suspension [Nexium] | No Availability Issues Detected |
| RXCUI: | 692576 |
|---|---|
| Dosage Form: | Granules for Oral Suspension |
| Dosage Route: | ORAL |
| Strength: | 20 |
| Measure: | mg |
| ANDA: | ANDA206055 |
| Generic Description: | ESOMEPRAZOLE MAGNESIUM DIHYDRATE |
| DEA: |